How Is Caustic Soda Made: A Comprehensive Guide to the Production Process
How Is Caustic Soda Made
Caustic soda, also known as sodium hydroxide (NaOH), is a versatile chemical compound used in industries ranging from paper manufacturing to water treatment. Understanding how caustic soda is made provides insight into one of the most important industrial processes in modern chemistry. In this article, we’ll explore the caustic soda production process step by step, including the primary methods, raw materials, and environmental considerations.
What Is Caustic Soda?
Caustic soda is a strong alkaline substance that appears as white flakes, pellets, or a concentrated liquid solution. It’s highly corrosive and soluble in water, producing heat upon dissolution. Chemically, it’s sodium hydroxide, and its production is closely tied to the chlor-alkali industry, where it’s generated alongside chlorine and hydrogen gas.

The global demand for caustic soda exceeds 80 million tons annually, driven by its role in soaps, detergents, textiles, and aluminum production. But how exactly is this essential chemical manufactured?
The History of Caustic Soda Production
The production of caustic soda dates back to the 19th century. Early methods involved the reaction of sodium carbonate (soda ash) with calcium hydroxide (slaked lime) in the Leblanc process. However, this was inefficient and polluting. Today, nearly all caustic soda (about 99.5%) is produced via the electrolytic chlor-alkali process, which evolved from mercury cell technology in the 1890s to more sustainable membrane cells in the late 20th century.
Primary Methods of Caustic Soda Production
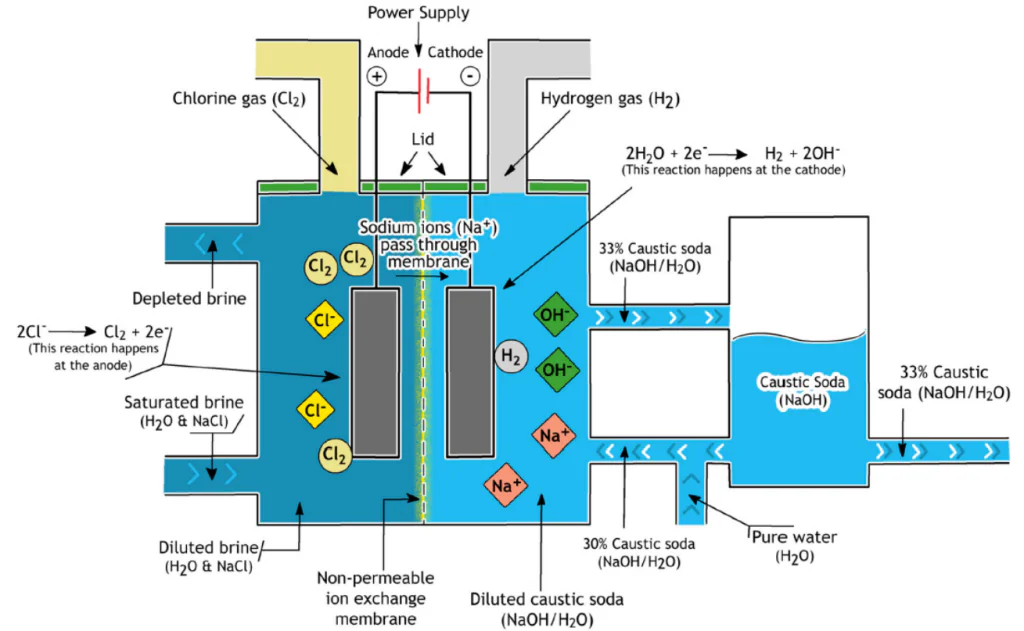
While historical methods like the lime-soda process exist, the dominant technique is electrolysis. There are three main electrolytic cell types: mercury, diaphragm, and membrane cells. The membrane cell is the most common due to its energy efficiency and lower environmental impact.
- Mercury Cell Process: In this older method, brine (sodium chloride solution) is electrolyzed using a mercury cathode. Sodium amalgam forms and reacts with water to produce caustic soda. It’s efficient but phased out in many regions due to mercury pollution.
- Diaphragm Cell Process: Uses an asbestos or polymer diaphragm to separate the anode and cathode compartments. It’s cost-effective but produces lower-purity caustic soda, requiring additional purification.
- Membrane Cell Process: The modern standard, employing an ion-exchange membrane to separate ions. It produces high-purity caustic soda with minimal energy waste and no hazardous byproducts like mercury.
The chlor-alkali process can be summarized by the equation: 2NaCl + 2H₂O → 2NaOH + Cl₂ + H₂
Step-by-Step Caustic Soda Production Process
The caustic soda production process begins with raw materials and ends with packaging. Here’s a detailed breakdown, focusing on the membrane cell method.
Step 1: Brine Preparation Sodium chloride (NaCl), or common salt, is dissolved in water to create brine. The brine is purified to remove impurities like calcium, magnesium, and iron, which could damage the electrolysis cells. This involves precipitation, filtration, and ion exchange.
Step 2: Electrolysis. The purified brine is fed into electrolytic cells. At the anode, chloride ions oxidize to form chlorine gas (Cl₂). At the cathode, water reduces to hydrogen gas (H₂) and hydroxide ions (OH⁻), which combine with sodium ions (Na⁺) passing through the membrane to form caustic soda (NaOH).

The process operates at 80–90°C with a voltage of 3–4 volts per cell. Modern plants use hundreds of cells in series for efficiency.
Step 3: Concentration and Purification The initial caustic soda solution is about 30–35% NaOH. It’s evaporated under vacuum to reach 50% concentration for liquid form or further dried to produce solid flakes or pellets. Mercury or other contaminants are removed if necessary.
Step 4: Byproduct Handling Chlorine and hydrogen are captured and used in other industries (e.g., PVC production). Waste heat is often recycled for energy savings.

Energy Efficiency and Environmental Impact
Caustic soda production is energy-intensive, consuming about 2.5–3.0 MWh per ton. Innovations like advanced membranes have reduced this by 30% since the 1970s. Environmental regulations have shifted away from mercury cells, with the EU banning them in 2017. Sustainable practices include recycling brine and using renewable energy sources.
Applications of Caustic Soda
Once produced, caustic soda is used in:
- Pulp and Paper: For bleaching and pulping.
- Textiles: In dyeing and mercerization.
- Water Treatment: To adjust pH and remove metals.
- Soaps and Detergents: As a key ingredient in saponification.
- Aluminum Production: In the Bayer process for bauxite refining.
Safety Considerations in Caustic Soda Handling
Caustic soda is highly corrosive, causing severe burns on contact. Production facilities adhere to strict safety protocols, including protective gear, ventilation, and spill containment. Storage requires compatible materials like stainless steel to prevent reactions.
Conclusion
The caustic soda production process, primarily through the chlor-alkali electrolysis method, is a cornerstone of industrial chemistry. As demand grows, advancements in membrane technology promise greener, more efficient manufacturing. Whether you’re in manufacturing or simply curious, understanding how caustic soda is made highlights the ingenuity behind everyday chemicals.
For more details on chemical processes, explore related topics like chlorine production or sustainable chemistry. (Contact Us)

